Repurposing a failed class of schizophrenia drugs to treat menopausal symptoms
The science and serendipity behind neurokinin-3 receptor antagonists
New treatments in medicine are often discovered by chance. This is the case for most psychiatric medications. The first antipsychotic, chlorpromazine, was originally developed as an anaesthetic. The first antidepressants were derived from anti-tuberculosis drugs. Lithium, the gold standard for bipolar disorder, was a 19th century treatment for gout.
All major classes of psychiatric drugs have relied on accidental but astute clinical observations. Rarely have they been designed with knowledge of underlying biology and there have been hardly any novel treatments (first in class) in the past 50 years.
Some have attempted more systematic methods of identifying compounds to repurpose, for example, using ‘big data’ from genetics or electronic health records. I’m not aware of any major successes from this approach, and there have been recent disappointments - statins do not appear effective for treatment resistant depression, the antibiotic minocycline failed in one RCT for first episode psychosis and in another for Alzheimer’s disease, and in another for bipolar depression.
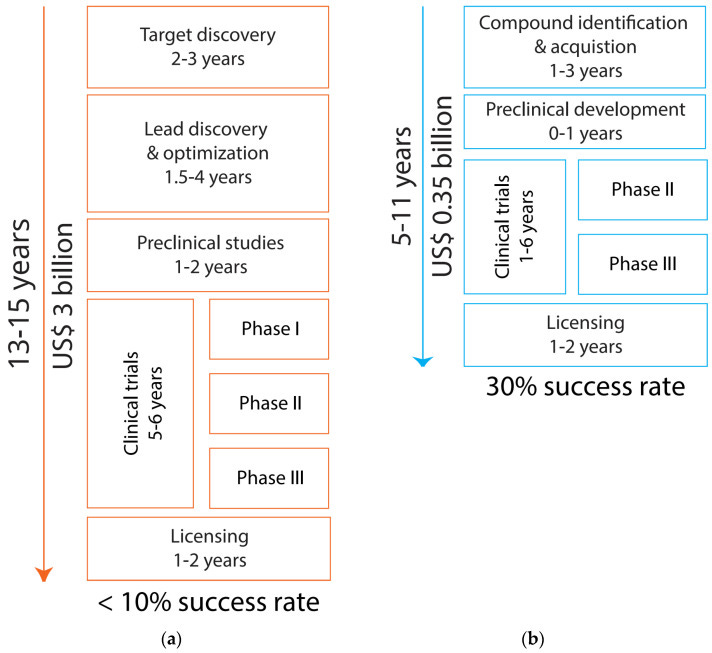
Nevertheless, repurposing is appealing for economic and clinical reasons. One estimate puts the cost of bringing a completely new molecule to market at $3 billion, over 13-15 years. Repurposing already available drugs might cut the time in half, at a fraction of the cost. Compared to creating new molecules, repurposed drugs have data on safety and what the drug actually does in human volunteers. For some drugs, they may even be licensed for another condition.
So while psychiatry looks for drugs to repurpose, one notable success goes in the other direction. This post describes neurokinin-3 receptor antagonists, medications that were initially developed (unsuccessfully) for schizophrenia but are now under FDA review for vasomotor symptoms associated with the menopause. It is a story of the painstaking study of biological mechanisms, with a little bit of luck along the way.
Vasomotor symptoms
Natural menopause is defined as 12 months of absent menstrual periods, without an identified pathological cause. It usually takes place between the ages of 45 and 55. Symptoms of the menopause have been underplayed but they impair the quality of life for a large proportion of women.
The menopause is associated with poor sleep, problems in concentration and memory, depressed mood, and vaginal dryness. However, the most frequent and troublesome symptoms for many are vasomotor symptoms (sometimes called hot flushes or hot flashes).
Vasomotor symptoms tend to manifest as a warm sensation on the neck, chest and face. This can be accompanied by sweating, palpitations and anxiety. During sleep, there may be drenching night sweats. They are caused by the brain mistakingly signalling to the body to lose heat by sweating and widening blood vessels near the skin (vasodilation).
Almost 80% of women going through the menopause experience vasomotor symptoms. For most, these last less than a year, but 1/3 continue to experience them fives years later. Unsurprisingly, these symptoms are associated with reduced quality of life, with 10-20% of women describing them as ‘intolerable’.
Although historically under-treated, they can be effectively controlled with HRT (Hormone Replacement Therapy) - usually a combination of transdermal oestradiol and oral progesterone. This greatly reduces the frequency of vasomotor symptoms.
There was previously a panic about the risks of HRT (particularly of breast cancer, blood clots and cardiovascular disease), following the 2002 Women’s Health Initiative study but for most individuals, a marginal increase in risk of certain diseases can be balanced against the relief of symptoms. Recent years have seen a marked increase in HRT prescriptions, suggesting its reputation has been restored.
While HRT is a safe and effective treatment for most women, there will always be a proportion who are unable to take it - for example, due to a history of breast cancer, active cardiovascular disease or blood clots. For others, the risk-benefit profile may be less certain - for example, with conditions that predispose to cardiovascular disease like diabetes, or in women who are at increased risk of familial breast cancer or blood clots. For this group, an alternative treatment is needed but current options (such as SSRIs or Clonidine) aren’t particularly effective.
Luckily new treatments are on the horizon that target the pathophysiology of vasomotor symptoms, in different way to hormone replacement.
Pathophysiology
Menopause
The physiology of the natural menopause is well described. It is a process resulting from reduced secretion of ovarian hormones (oestradiol and progesterone), due to the depletion of the finite reserve of oocytes. The usual negative feedback loops governing the hypothalamic-pituitary-gonadal axis become unbalanced - decreasing circulating levels of ovarian hormones result in less inhibition, so levels of FSH and LH rise.

The menstrual cycle becomes irregular. Anovulatory cycles (in which no egg is released) become more common. During the perimenopause, ovarian hormones can widely fluctuate, showing a more consistent decline following cessation of menstruation.
Since the menopause is characterised by declining levels of ovarian hormones, it seems logical to relieve associated symptoms by hormone replacement. As discussed, this is effective for treating vasomotor symptoms, but will not be suitable for all and will depend on each individual’s risk-benefit profile.
To devise new treatments which do not involve simply replacing ovarian hormones, we need to delve deeper - to understand not just the hormonal changes associated with the menopause, but the mechanisms behind vasomotor symptoms. To do so, we will zoom in on one part of the hypothalamic-pituitary-gonadal axis, the hypothalamus.
Temperature dysregulation
Literally meaning ‘below the thalamus’, the hypothalamus is the temperature regulation centre of the brain. Unlike the physiology of ovarian hormones, the mechanisms of temperature dysregulation are only recently being uncovered, thanks mostly to a team led by Professor Naomi Rance at the University of Arizona.
They studied a small population of cells in the hypothalamus which express the neuropeptides kisspeptin, neurokinin B, and dynorphin and are therefore known as KNDy (pronounced candy) neurones. These neuropeptides signal the body to activate its heat loss mechanisms of vasodilation and sweating.
Hypertrophy (enlargement) of this cell population is seen in postmortem samples from women following menopause. In monkeys, removing the ovaries leads to hypertrophy of KNDy cells which is reversed by oestrogen replacement
In rats, Rance’s team studied what happened when these neurones were destroyed using a selective toxin and demonstrated that the rats had absent vasodilatation. In keeping with these findings, KNDy neurones were found to project to areas of the brain (such as the median pre-optic nucleus of the hypothalamus) that control heat regulation. Furthermore, activating neurokinin-3 receptors in this area cooled the rats body temperature.
Another important finding was that inactivating these KNDy neurones did not seem to do the rats harm. This posed an intriguing possibility - that the vasodilation caused by KNDy neurones due to low ovarian hormones might be switched off without major problems in the organism’s physiology. Could a similar mechanism be used to inhibit vasomotor menopausal symptoms?
In 2013, in a culmination of many years of work, Rance published her hypothesis that KNDy neurones, acting on neurokinin-3 receptors, generate the vasodilation that causes menopausal vasomotor symptoms. Two years later, a team from Imperial College London, led by Professor Waljit Dhillo demonstrated that neurokinin B administered to pre-menopausal women caused vasomotor symptoms.
Within four years of Rance publishing her hypothesis, a positive phase II randomised controlled crossover trial using a neurokinin-3 receptor antagonist was published in the Lancet by the endocrinologist Dr Julia Prague (under the supervision of Dhillo) - reducing the frequency of vasomotor symptoms in menopausal women by 73% compared with baseline and by 45% compared with placebo.
The relative speed going from finding the neurones responsible for these symptoms to having medications primed for clinical use is the serendipitous part of this story.
Neurokinin-3 receptor antagonists
The leap from target discovery to phase II trial was easy to make, as neurokinin-3 receptor antagonists had already been developed, though not as a treatment for menopause. A 2005 Nature Reviews Drug Discovery article, speculated, that neurokinin-3 receptor antagonists could be the next generation of antipsychotics.
Expression of neurokinin-3 receptor in areas important for schizophrenia, such as the hippocampus, suggested they may be implicated in this disorder. Furthermore, dopaminergic neurones in the midbrain were found to increase activity in response to a neurokinin-3 receptor agonists and to be inhibited by an antagonist. (The dopamine hypothesis is the leading neurobiological explanation of antipsychotic action and psychotic symptoms, putting increased dopaminergic activity at the heart of schizophrenia.)
It would have seemed logical to block the neurokinin-3 receptor in an attempt to relieve psychotic symptoms, while avoiding some of the side-effects associated with direct dopamine antagonism. While initial clinical trials were promising, by 2012 development was discontinued as all three antagonists, Osanetant, Talnetant, and Pavinetant, failed to beat placebo in phase II trials.
These medicines were therefore shelved for schizophrenia, but this meant that when the role of neurokinin B in vasomotor symptoms was discovered, they were already there… waiting to be lifted back off the shelf.
Dhillo had been working on how neurokinin B influences gonadotrophin releasing hormone and noticed it had caused flushing in participants. By chance, he attended a talk by Rance in 2012, where she presented her work on KNDy neurones. He later said if he hadn’t heard the talk, he may have missed her paper when it came out.
He knew neurokinin-3 receptor antagonists were on the books of various pharma companies in need of a clinical application. He approached Astra-Zeneca who agreed to provide Pavinetant for his 2017 Lancet Trial. Pavinetant was later discontinued, this time due to safety concerns around liver injury, but the principle had been proven. Since then, positive trial results for other neurokinin-3 receptor antagonists have come thick and fast.
Fezolinetant, from Astellas Pharma, recently published findings from two phase III trials, SKYLIGHT 1 and SKYLIGHT 2, with each meeting all primary endpoints in reducing the severity and frequency of vasomotor symptoms. A one year follow-up of 1,800 participants for safety monitoring, SKYLIGHT 4, has reported on ClinicalTrials.gov, but has not yet been published in a peer-reviewed journal. These studies will form the basis of regulatory submission in the US and Europe, with a decision from the FDA expected in Summer 2023. Curiously, Astellas announced disappointing results from its phase III trial in Asia, MOONLIGHT 1, failing to meet primary endpoints. Without the full results being published, it’s difficult to speculate as to why this one was negative.
Another compound, Elinzanetant, has been developed by Bayer with dual neurokinin-1 and -3 receptor antagonism. They have recently published positive results from a phase IIb trial, SWITCH-1, with phase III trials underway.
Relevance for psychiatry
Will the re-emergence of neurokinin-3 receptor antagonists have direct relevance for mental disorders? My hunch is probably not. We already know they probably won’t be effective for disorders like schizophrenia. There might be an inkling to trial them in menopause related disorders like perimenopausal depression or schizophrenia with onset in midlife. However, the finding that these drugs may lower gonadal hormones goes against our understanding of how depression and schizophrenia are influenced by such hormones. Monitoring depressive symptoms in randomised trials of these drugs could give an early indication if this is worth pursuing.
More broadly, I hope this will provide impetus to probe neuroendocrine mechanisms behind mental disorders, in search for novel drug targets. In my introduction, I stated that very few psychiatric medicines have successfully been brought to market through knowledge of the underpinning biology. Well, one rare success is Brexanolone (allopregnanolone) for postpartum depression. Perhaps a focus on other hormonal causes of mental disorders might bring more in its wake?





Looks like Veozah caused liver injury in someone (https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-warning-about-rare-occurrence-serious-liver-injury-use-veozah-fezolinetant-hot-flashes-due). Makes me wonder if that's a risk of neurokinin-3 receptor antagonists. I'm guessing Bayer will be worried about that as well.